Marginal Bone Changes on Ultraclean, Micro-Threaded Platform-Switched Implants Following Restoration: 1- to 4-Year Data
Michael Klein, DDS; Dennis Tarnow, DDS; and Lauren Lehrfield, BSc, RDH
Abstract
Objective: The aim of this clinical study was to retrospectively evaluate changes in bone following final abutment insertion and functional loading and to evaluate bone status relative to implant type, width, and length; placement into healed bone and extraction sockets; immediate provisionalization; abutment type (single-unit, multi-unit, cementable stock abutment, custom abutment, ti-base, UCLA); cementable restoration, screw-retained restoration, splinted restoration, and single-unit restoration. Materials and Methods: Fifty consecutive patients with 87 implants were evaluated radiographically following final abutment insertion and functional loading to their latest follow-up radiograph. Follow-up evaluation time from final abutment insertion ranged from 11 months (335 days) to 4 years (1,484 days), with an average 1`e of 831 days (2.3 years). Mesial and distal surfaces were examined and graded as bone improved, bone maintained, and bone decreased. A total of 174 surfaces were graded (87 implants). Results: Thirty percent of implant surfaces showed bone improvement following restoration, 62% of implant surfaces showed bone maintenance, and 8% showed bone decrease (range 0.1 mm to 1 mm). Conclusions: This retrospective study showed an unusual phenomenon of bone improvement following restoration for 30% of implant surfaces. Eight percent of the surfaces showed bone decrease but at a maximum of 1 mm. This places 100% of the followed implants well within established criteria for successful implant bone maintenance. There was no statistical difference among the groups in age, gender, implant diameter, implant length, implant location (maxilla versus mandible, anterior versus posterior), and prosthetic procedures. Additional highly controlled prospective studies are being planned to validate and further the authors' knowledge
Bone is and has been a primary consideration in dental implant treatment. Adequate bone, determined by analysis of its height, width, and density as well as forces to be placed on the implant, is required at the time of implant placement to provide for the critical criterion ofinitial implant stability. Without sufficient bone to stabilize the implant, micromovement may occur and result in a fibrous tissue interface with the implant.1,2 Equally important is bone maintenance at the implant-to-bone interface over time following prosthetic restoration of the implant. Minimum standards were set many years ago for what was considered acceptable bone loss over time and at what rate. These standards were established based on what was observed to occur to a commercial, pure titanium machined-surface implant over time. It was accepted that up to 2 mm of initial bone loss would occur in the first year of restoration, followed by continuing bone loss of no more than 0.2 mm per year thereafter.3
One has to question if these criteria are still appropriate or acceptable for the current state of implant surface-roughened micro-pitted design. Different implant manufacturers have used different geometric design strategies and implant surface treatment technologies in efforts to create faster implant integration to bone, reduce initial crestal bone loss, and manage bone maintenance over time. More recent studies of implants with a platform-switch design have shown less bone loss and better bone maintenance over time,4,5 but there are no accepted criteria for platform-switched implants. The initial strategy of incorporating a roughened micro-pitted surface to increase the rate of bone integration seems to have been accomplished according to manufacturer reports.6,7 Of more significance is the subsequent strategy of reducing initial crestal bone loss and preventing progressive bone loss around implants. The development of peri-implantitis has been reported in the range of 10% to 50% of implants depending on the criteria used to determine true peri-implantitis.8,9 This is an alarming statistic. The exposure of the roughened implant surface creates an environment that initiates peri-implantitis by creating a haven for bacteria that is not cleansable. The ensuing bacterial endotoxins result in an inflammatory process that leads to the breakdown of bone with subsequent bone loss.10,11
Multiple strategies have been presented for managing and trying to eliminate peri-implantitis and recover bone whenever possible. These strategies have limited and unpredictable long-term success due to many variables.
Purpose
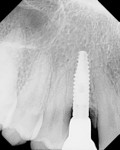
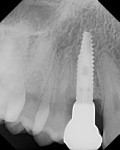
The aim of this study was to evaluate bone changes on an implant system (Paltop Implant System, Paltop Advanced Dental Solutions Ltd/Keystone Dental Inc, paltopdental.com) with an ultrapure sandblasted microetched titanium alloy surface (2 µm to 3 µm roughness) that covers the platform switch, on consecutive cases in a single practitioner's office following final abutment insertion and functional loading. This retrospective study evaluates the incidence of bone changes over time and attempts to identify the factors that affect these bone changes (Figure 1 and Figure 2).
Materials and Methods
Study Design
Fifty consecutive patients who met eligibility and radiographic criteria were evaluated in this study. All 50 patients had surgical treatment provided by the same surgeon; multiple restorative doctors restored the patients. All implants placed were manufactured by the same manufacturer (Paltop Advanced Dental Solutions Ltd). All implants were made from titanium alloy (Ti6AL4V ELI) and had surface treatment that consisted of sandblasting with large-grit aluminum oxide and acid-etching with phosphoric acid (commonly referred to as SLA). The resulting surface roughness was 2 µm to 3 µm. The implant-to-abutment interface connection was an internal hex with platform shifting. Three styles of external geometric implant body configurations were used in the study, but all had the same coronal design (microthreads, platform switch, and microtextured surface on the platform of the implant).
Implant bone levels were evaluated from the time of final abutment insertion and functional loading. Evaluation was done comparing periapical radiographs over a minimum of 11 months following final abutment insertion. Data recorded included patient sex, age, date of surgery, date of abutment insertion, dates of all follow-up x-rays, and style of implant (Paltop Advanced, Paltop Advanced +, Paltop Dynamic). All of these implant styles have the same connection, coronal microthread design, overall external geometry, and surface treatment. The differences are the Advanced style has a round implant apex, the Advanced + has one apical cutting thread, and the Dynamic has more aggressive cutting threads throughout the length of the implant body except for the microthreads. Factors evaluated included implant diameter (3.25 mm, 3.75 mm, 4.2 mm, 5 mm) and implant length (8 mm, 10 mm, 11.5 mm, 13 mm, 16 mm); implant placement into healed bone, implant placement with bone graft, implant placement with tooth extraction and bone graft, implant placement into previous bone graft, implant placed adjacent to another implant placed either at the same time or a different time, implant buried (two-stage) surgical placement, implant placed with the healing abutment (one-stage implant surgical placement); immediate provisionalization; abutment connection (narrow platform, standard platform); style of abutment (single-unit abutment, multi-unit abutment, ti-base abutment, stock cementable abutment, custom abutment, UCLA abutment); and screw-retained or cementable restoration, splinted restoration, and individual unit. The placement of implants in thick- versus thin-biotype patients was not a measurement that was taken.
Study Population and Eligibility Requirements
The study included 50 patients; 27 were female, 23 were male. The age range was 19 to 93 years at the time of restoration. Patients with uncontrolled diabetes or on long-term steroid usage were excluded. Patients with a history of or actively on bisphosphonate therapy were included in the study data.
Radiographic Eligibility Criteria
No system was used to standardize x-rays, thereby limiting the conclusions that could be made. Criteria were created to qualify x-rays for comparison.
Radiographs to be included were periapical radiographs of good quality with reasonable expectation of parallelism. The assessment of reasonable parallelism was made based on evaluation of the microthreads on the implants for any appearance of elongation or foreshortening. All x-rays were taken using the Dexis system (Dexis Classic sensor, DEXray release 9.4.3, Kavo Kerr, dexis.kavokerr.com) utilizing paralleling devices Only x-rays taken from the same orientation/positioning were included. Only patients who had periapical x-rays after final abutment insertion with an at least 11-month follow-up x-ray that fulfilled these criteria were included in the study.
Radiographic Measurements
All radiographs were evaluated in the same manner. X-rays were displayed on a 24-inch high-definition monitor. The mesial and distal surfaces of the implant body were evaluated from the point of connection (coronal end of the platform switch) to the most coronal point of bone. This was done on the first x-ray following final abutment insertion and repeated on the last follow-up x-ray available.
For each implant each surface (mesial and distal) of the initial post-abutment x-ray was compared to the corresponding surface on the last x-ray available. The comparison was done by evaluating and counting easily identifiable points such as the coronal and apical position of the platform switch and by counting microthreads. The implant surface was then graded as "improved," "same," or "decreased." The decreased surfaces were measured; due to the lack of standardization of the x-rays, however, these measurements could not be assessed as accurate and for these same reasons the amount of improvement was not measured (concerns of elongation and foreshortening causing misinterpretation of bone position, however, would be represented by both gain and loss of bone).
A control implant group was also analyzed utilizing the same criteria and techniques. The implant design in the control group was a tapered threaded implant with an external surface roughened with a resorbable blast media and treated with acid and had coronal microchannels and an internal hex connection. The control group consisted of 10 patients with 23 implants. The follow-up evaluation was done from 1 year to 5 years 7 months from restoration of the implants.
Results
The data was compiled and analyzed for the following: implants that had bone improvement versus bone maintenance versus bone decrease; male patients versus female patients; varying implant diameters and lengths; placement into extraction sockets versus healed bone versus healed bone with a previous bone graft; immediate healing abutment placement versus immediate provisional placement versus buried implant; flap versus flapless technique; screw-retained restoration versus cementable restoration; single-unit restoration versus splinted units; and abutment style: multi-unit versus single-unit versus stock cementable versus ti-base versus UCLA.
Implant Position by Tooth Number
The study analyzed 87 implants in 50 patients-27 females and 23 males-measuring 174 surfaces (mesial and distal of each implant). Implants included 83 Paltop Advanced implants, three Paltop Advanced + implants, and one Paltop Dynamic implant. The length of time from final abutment placement to the last x-ray analyzed was 11 months (335 days) to 4 years (1,484 days). There were nine implants of 3.25 mm diameter, 25 of 3.75 mm diameter, 28 of 4.2 mm diameter, and 25 of 5 mm diameter. There were eight implants of 8 mm length, 13 of 10 mm length, 18 of 11.5 mm length, 44 of 13 mm length, and four of 16 mm length.
A breakdown of implant placement is as follows:
• 41 implants were placed in the maxilla, 46 were placed in the mandible (control group: 18 in maxilla, five in mandible).
• 46 implants were placed in healed bone with no previous bone graft (control group: 12 such implants).
• 19 implants (not including three implants placed into previous sinus grafts) were placed into healed bone that had a previous bone graft. In 18 of these sites the bone grafts were mineralized allografts, 10 of those sites had regenerative resorbable collagen membranes placed over the allograft. One site had one implant placed into a previously healed autogenous monocortical bone block placed laterally to increase ridge width (control group: five implants placed with simultaneous bone grafts).
• 22 implants were immediately placed in extraction sockets. All extraction sockets with immediate implant placement had implants placed toward the lingual of the socket, leaving at least 2 mm of space between the buccal plate and the buccal external implant body. A xenograft was placed into any remaining space between the implant and bony walls of the extraction socket. The xenograft was placed level with the coronal end of the implant platform. The implants in extraction sockets were placed 1 mm to 2 mm below the buccal alveolar crest (control group: six such implants).
• 16 implants were placed with a flapless implant placement technique (three of these implants were immediately loaded, 13 were in extraction sockets, and three were done with soft-tissue trephines); 71 implants were placed using open-flap procedures. 22 implants were buried, 50 implants were done with one-stage procedures with healing abutments, 11 of these implants were immediately loaded with temporary restorations.
• 14 implants were immediately loaded with temporary restorations (control group: eight such implants).
• There were five implants placed into four healed sinus lifts, three implants placed into two sinus lifts during sinus lift procedure, and three implants placed into simultaneous sinus osteotome procedures. (The implants placed into sinus lifts or sinus osteotome procedures were not counted in the previous bone graft category or simultaneous bone graft because no grafting was done around the coronal aspect of the implant.)
Status of Bone Surfaces Post Restoration
Bone changes post-restoration were determined by analyzing bone levels adjacent to the mesial and distal implant surfaces. A total of 87 implants with 174 surfaces were analyzed, and the following was determined: 52 surfaces showed improvement of the bone level, 109 surfaces maintained their bone level, and 13 surfaces showed a decrease in bone level. The decrease measured 0.1 mm to 1 mm (not considered a precise measurement due to the lack of absolute standardization of the x-rays). Bone increase was found on 30% of surfaces, bone maintenance on 62% of surfaces, and bone decrease on 8% of surfaces. (Control group: 23 implants, 42 surfaces; two surfaces showed bone increase [4.76%], 26 surfaces showed bone maintenance [61.9%], and 14 surfaces showed bone decrease [33.33%]).
Bone Status by Patient Gender
The patient makeup was 27 females and 23 males. On female patients, 21 surfaces showed bone improvement, 67 showed bone maintenance, and eight showed bone decrease. On male patients, 31 surfaces showed bone improvement, 42 showed bone maintenance, and five showed bone decrease (Table 1).
Bone Status by Implant Placement Into Extraction Sockets Vs Healed Bone Vs Healed Bone With a Previous Bone Graft
Nineteen implants were placed into healed bone with a previous bone graft, 46 were placed into healed bone, and 22 were placed into extraction sockets. In healed bone with a previous bone graft 16 surfaces showed bone improvement, 21 surfaces showed bone maintenance, and one surface showed bone decrease. In healed bone 23 surfaces showed bone improvement, 60 surfaces showed bone maintenance, and nine surfaces showed bone decrease. In extraction sockets 13 surfaces showed bone improvement, 28 surfaces showed bone maintenance, and three surfaces showed bone decrease (Table 2).
Bone Status by Immediate Healing Abutment Vs Immediate Temporary Vs Buried
There were 51 implants placed with an immediate healing abutment, 14 implants were placed with an immediate temporary, and 22 implants were buried. For implants placed with an immediate healing abutment 39 surfaces showed bone improvement, 57 surfaces showed bone maintenance, and six surfaces showed bone decrease. For implants placed with an immediate temporary 10 surfaces showed bone improvement, 12 surfaces showed bone maintenance, and six surfaces showed bone decrease. For implants that were buried, three surfaces showed bone improvement, 40 surfaces showed bone maintenance, and one surface showed bone decrease (Table 3).
Bone Status by Flap Vs Flapless
There were 71 implants placed with a flap during surgery, and 16 placed flapless during surgery. In procedures performed with a flap, 39 surfaces showed bone improvement, 93 surfaces showed bone maintenance, and 10 surfaces showed bone decrease. In procedures performed without a flap (flapless), 13 surfaces showed bone improvement, 16 surfaces showed bone maintenance, and three surfaces showed bone decrease (Table 4).
Bone Status by Implant Diameter
In the study group there were 18 implant surfaces of 3.25 mm diameter, 50 implant surfaces of 3.75 mm diameter, 56 implant surfaces of 4.2 mm diameter, and 50 implant surfaces of 5 mm diameter.
For 3.25 mm diameter implants, four surfaces showed bone improvement, 10 surfaces showed bone maintenance, and four surfaces showed bone decrease. For 3.75 mm diameter implants, 10 surfaces showed bone improvement, 38 surfaces showed bone maintenance, and two surfaces showed bone decrease. For 4.2 mm diameter implants, 20 surfaces showed bone improvement, 31 surfaces showed bone maintenance, and five surfaces showed bone decrease. For 5 mm diameter implants, 18 surfaces showed bone improvement, 30 surfaces showed bone maintenance, and two surfaces showed bone decrease (Table 5).
Bone Status by Implant Length
There were 16 implant surfaces of 8 mm in length, 26 implant surfaces of 10 mm in length, 36 implant surfaces of 11.5 mm in length, 88 implant surfaces of 13 mm in length, and eight implant surfaces of 16 mm in length.
For 8 mm length implants, bone improved on 10 surfaces, bone maintained on six surfaces, and bone decreased on zero surfaces. For 10 mm length implants, bone improved on 10 surfaces, bone maintained on 14 surfaces, and bone decreased on two surfaces. For 11.5 mm length implants, bone improved on four surfaces, bone maintained on 25 surfaces, and bone decreased on seven surfaces. For 13 mm length implants, bone improved on 25 surfaces, bone maintained on 60 surfaces, and bone decreased on three surfaces. For 16 mm length implants, bone improved on three surfaces, bone maintained on four surfaces, and bone decreased on one surface (Table 6).
Bone Status by Cementable Vs Screw-Retained Restoration
There were 24 cement-retained restorations and 63 screw-retained restorations. For cementable restorations, 17 surfaces showed bone improvement, 27 surfaces showed bone maintenance, and four surfaces showed bone decrease. For screw-retained restorations, 35 surfaces showed bone improvement, 82 surfaces showed bone maintenance, and nine surfaces showed bone decrease (Table 7).
Bone Status by Single-Unit Restoration Vs Splinted Restoration
For single-unit restorations (49 restorations) there were 98 surfaces, and for splinted restorations (38 restorations) there were 76 surfaces. Among the single-unit restorations, 31 surfaces showed bone improvement, 56 surfaces showed bone maintenance, and 11 surfaces showed bone decrease. Among the splinted restorations, 21 surfaces showed bone improvement, 53 surfaces showed bone maintenance, and two surfaces showed bone decrease (Table 8).
Bone Status by Abutment Type
There were 40 custom abutment surfaces, 68 multi-unit abutment surfaces, 12 cementable stock abutment surfaces, 24 ti-base abutment surfaces, and 30 UCLA abutment surfaces.
For custom abutments, 12 surfaces showed bone improvement, 25 surfaces showed bone maintenance, and three surfaces showed bone decrease. For multi-unit abutments, 16 surfaces showed bone improvement, 50 surfaces showed bone maintenance, and two surfaces showed bone decrease. For cementable stock abutments, six surfaces showed bone improvement, five surfaces showed bone maintenance, and one surface showed bone decrease. For ti-base abutments, 12 surfaces showed bone improvement, 10 surfaces showed bone maintenance, and two surfaces showed bone decrease. For UCLA abutments, six surfaces showed bone improvement, 19 surfaces showed bone maintenance, and five surfaces showed bone decrease (Table 9).
Timespan Between Final Abutment Insertion and Final Follow-up X-ray
A breakdown of bone status by timespan between final abutment insertion and final follow-up x-ray is provided in Table 10. A recording of number of implants in the timespan between final abutment insertion and final follow-up x-ray is shown in Table 11.
Statistical Analysis
Because of the limited number (N) of subjects in this study, not enough data was available to perform a statistical analysis; only trends could be analyzed. One trend was that for the surfaces that showed bone increase, there was no difference between immediate implant placement in extraction sockets and implant placement in healed ridges. Implants placed in healed ridges with previous bone grafting had a greater tendency to bone increase. Other identifiable trends were that the small number of decreased surfaces occurred in healed ridges without previous bone grafts, and implants placed in flapless procedures had a greater tendency to bone improvement.
There was no statistical difference among the groups in age, gender, implant diameter, implant location (maxilla versus mandible, anterior versus posterior), and prosthetic procedures (immediate load, abutment type, restoration design).
Discussion
This critical analysis on 50 implant patients with 87 implants placed evaluated bone changes (ie, movement) following final abutment insertion. Implants functionally loaded with their final restorations were followed for a period ranging from 11 months to 4 years, with an average of 831 days (2.3 years). The analysis was done through radiographic comparison with limitations previously described (that is, foreshortening and elongation can cause misinterpretation of bone movement but would indicate decreased bone as easily as increased bone). Bone improvement was identified 30% of the time, bone maintenance 62% of the time, and bone decrease 8% of the time. Bone decrease ranged from 0.1 mm to 1 mm. This amount of bone decrease falls well within the standards set for implants. In this study, 100% of implant surfaces were within the bone level standards set by Albrektson and Zarb.3
Surgical and restorative factors that may have had an effect on bone movement were analyzed. These categories were segmented as follows: patient age, gender, maxilla versus mandible, anterior versus posterior implant position, implant diameter, implant length, implant placement into immediate extraction sockets versus healed bone versus healed bone with previous augmentation, one-stage surgery (placement of immediate healing abutment) versus two-stage surgery (buried implant) versus immediate provisionalization, screw-retained restoration versus cementable restoration, single units versus splinted units, and comparison of abutment styles (multi-unit, single-unit, stock cementable abutment, ti-base, custom abutment/CAD/CAM, UCLA).
Because of the limited number of subjects in this study only trends could be examined and no statistical significance between the groups could be determined. The trends that could be seen for the surfaces that showed bone increase were that there was no difference between immediate implant placement in extraction sockets and implants placed in healed ridges, and implants placed in healed ridges with previous bone grafting had a greater tendency to bone increase. Other identifiable trends were that the limited number of decreased surfaces occurred in healed ridges without previous bone grafts and implants placed in flapless procedures had a greater tendency to bone improvement. All of these measurements were made at insertion of the final prosthesis and not at the time of the surgical procedure.
There was no statistical difference among the groups in age, gender, implant diameter, implant location (maxilla versus mandible, anterior versus posterior), and prosthetic procedures (immediate load, abutment type, restoration design).
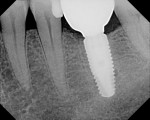
The finding of bone improvement following restoration is an unusual, unexpected phenomenon, and it is postulated that the design, manufacturing processes, high surface purity, machining tolerances, transgingival abutment design, and platform switching with an etched shoulder contributed to this phenomenon of bone improvement following restoration (Figure 3 and Figure 4).
A control group of implants with some common characteristics and a similar restored timeframe to the analyzed implants was also evaluated. The overall data showed 33.3% of surfaces had bone decrease, 61.9% of surfaces maintained their bone, and 4.76% of surfaces had bone increase. The sample size was fairly small to make any tangible conclusions relative to the control implant; however, it is clear that the positive bone movement or appearance seen with the implants in this study was not obtained with the control implant with the same clinicians using the same evaluation criteria.
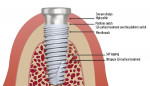
The implant system used in this study (Paltop Implant System) includes a titanium alloy implant with a geometric design that incorporates a tapered apical section, a straight-walled mid-section, microthreads on the coronal section, and platform switching at the implant connection level. The surface treatment comprises a proprietary ultrapure sandblasting, acid-etching technique that covers the shoulder of the platform switch. The connection is designed with very close tolerances. The abutment system is designed with a concave transgingival geometry (Figure 5).
Possible Reasons for Observations of Bone Improvement
A number of hypotheses could be presented to possibly explain why bone improvement occurred:
Platform switching: Platform switching has been shown to support bone maintenance and even encourage bone growth. The platform switch moves the junction of the connection away from the bone-to-implant interface. This is thought to aid in bone maintenance by providing space for the development of biologic width, which begins at the abutment connection. By moving the connection away from the implant-to-bone interface, remodeling of the bone is limited. It is also thought that any potential bacterial leakage through the abutment connection is distanced from the implant-to-bone interface by the platform switch.12,13
Sandblasting and acid-etching surface treatment (commonly referred to as SLA) covering the platform switch: SLA covering the platform switch encourages bone growth to the abutment connection.14,15
Ultrapure surface treatment: The term SLA describes an overall surface treatment concept. However, the actual specific process can and often does differ from implant manufacturer to manufacturer. The process of degreasing the implant after machining, followed by aluminum-oxide blasting, to create a roughened implant surface is common to manufacturers. Passivation is accomplished in a nitric acid bath followed by micro-pitting with phosphoric acid. Finally, all of the impurities from this process are cleaned from the implant surface with water. There are many variations within these steps from implant manufacturer to implant manufacturer. These variations result in residual surface impurities found on finished packaged implants.16 The implant system used in this particular study was found to have an ultrapure surface free of impurities.16
Titanium and titanium alloy are the material of choice for dental implants due to their ability to readily passivate and form a strong oxide layer.17-19 This oxide layer prevents corrosion by helping maintain the chemical bonds of the surface titanium. Impurities on the surface of the titanium create holes in the oxide layer.20These holes possibly allow greater release of titanium ions.21-23 Titanium ions in the gingival tissue around implants have been theorized to prevent the precursor cells of Langerhans cells from differentiating into Langerhans cells. It has been found that there is a significant deficiency of Langerhans cells around dental implants (in the mouse model).24 Langerhans cells are known to provide the baseline antibody response to bacteria around teeth.25,26 The absence of Langerhans cells would therefore allow a more significant inflammatory response to bacteria around dental implants. Thus, a possible theory may be that an implant surface free of all impurities will have an intact oxide layer, thereby having less titanium ion release. If there is less titanium ion release, then a greater presence of Langerhans cells should be present, resulting in a better antibody response to bacteria with less inflammation around dental implants.
Microthreads: The design geometry of microthreads at the coronal end of an implant is a popular external design feature. It is commonly believed that the microthreads reduce stress to the cortical bone, thereby supporting bone maintenance.27,28 Although this theory is debatable,29 microthreads do increase the surface area of the implant by virtue of their peaks and valleys,30 which alone provides value to the microthread design.
Concave transgingival abutment design: The implant design itself is just one component of a system that maintains and promotes bone growth at the level of the implant connection. The gingival complex that surrounds the abutment at the level of the implant-abutment connection contributes significantly to a system of bone protection.31,32 A robust gingival apparatus can help minimize the possible inflammatory response around the implant abutment.33,34 A tight seal around the abutment helps prevent bacterial invasion and penetration through the abutment sulcus, which may be a primary cause of bone breakdown due to the inflammatory response prompted by the bacterial infiltrate.35 The geometrical design of the abutment also has been proposed as a possible cause of bone loss due to bone remodeling in an effort to establish a peri-implant biologic width.36 The transgingival concave design of the abutment addresses both of these issues.
Close mechanical tolerances in the abutment connection design: Close tolerances enhance the seal of the implant-abutment connection. Minimizing the potential for any micromovement in this connection facilitates an improved seal against bacterial penetration through the connection, thereby helping to prevent an inflammatory response around the implant. The engineering design of the implant-to-abutment connection has a point of contact like in a conical connection as opposed to a flat surface to flat surface. This point of contact creates an effective seal on the implant-to-abutment connection, which is the point of entry for bacteria.37,38
Conclusions
Limited bone loss monitored over time has been accepted as the standard for adequately healthy implants. However, the incidence of peri-implantitis being reported is alarming. Perhaps this is due to applying criteria for machined implant surfaces to roughened surfaces. An implant system with a combination of design geometries and critical manufacturing processes has been observed over time to offer bone improvement. A preliminary effort has been made to identify critical causal factors. Due to the limited number of patients, implants, and clinicians in this study only trends could be evaluated. In addition, standardized x-rays were not used, although x-rays were qualified to be included for evaluation. While consideration must be given for angulation of x-rays, it is clear that positive bone movement is occurring in many cases where mostly bone loss has been seen on older implant designs. Additional multicenter prospective studies are required and underway to confirm and validate these findings and identify critical causal factors for bone improvement.
Disclosure: Dr. Klein is Medical Director of Keystone Dental Inc./Paltop Advanced Dental Solutions Ltd. Dr. Tarnow is a consultant to Keystone Dental Inc. Ms. Lehrfield is an employee at Paltop Advanced Dental Solutions, Ltd.
Acknowledgment: The authors thank Hanae Saito, DDS, MS, CDC, for her assistance in analyzing and reviewing the statistics for this study.
About the Authors
Michael Klein, DDS
Medical Director, Keystone Dental Inc./Paltop Advanced Dental Solutions Ltd.; Private Practice, Cedarhurst, New York
Dennis Tarnow, DDS
Clinical Professor, Department of Periodontology, Director of Implant Education, Columbia University, College of Dental Medicine, New York, New York
Lauren Lehrfield, BSc, RDH
QA & RA Associate, Paltop Advanced Dental Solutions, Ltd.
References
1. Zarb CA, Albrektsson T. Nature of implant attachments. In: Brånemark PI, Zarb CA, Albrektsson T, eds. Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry. Chicago, IL: Quintessence Publishing; 1985:88-98.
2. Lioubavina-Hack N, Lang NP, Karring T. Significance of primary stability for osseointegration of dental implants. Clin Oral Implants Res. 2006;17(3):244-250.
3. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986;1(1):11-25.
4. Pan YH, Lin HK, Lin JC, et al. Evaluation of the peri-implant bone level around platform-switched dental implants: a retrospective 3-year radiographic study. Int J Environ Res Public Health. 2019;16(14):E2570.
5. Cappiello M, Luongo R, Di Iorio D, et al. Evaluation of peri-implant bone loss around platform-switched implants. Int J Periodontics Restorative Dent. 2008;28(4):347-355.
6. Barfeie A, Wilson J, Rees J. Implant surface characteristics and their effect on osseointegration. Br Dent J. 2015;218(5):E9.
7. Deligianni DD, Katsala N, Ladas S, et al. Effect of surface roughness of the titanium alloy Ti-6Al-4V on human bone marrow cell response and on protein adsorption. Biomaterials. 2001;22(11):1241-1251.
8. Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol. 2008;35(8 suppl):286-291.
9. Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42(suppl 16):S158-S171.
10. Mellado-Valero A, Buitrago-Vera P, Solá-Ruiz MF, Ferrer-García JC. Decontamination of dental implant surface in peri-implantitis treatment: a literature review. Med Oral Patol Oral Cir Bucal. 2013;18(6):e869-e876.
11. Heitz-Mayfield LJ. Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol. 2008;35(8 suppl):292-304.
12. Atieh MA, Ibrahim HM, Atieh AH. Platform switching for marginal bone preservation around dental implants: a systematic review and meta-analysis. J Periodontol. 2010;81(10):1350-1366.
13. Enkling N, Jöhren P, Klimberg V, et al. Effect of platform switching on peri-implant bone levels: a randomized clinical trial. Clin Oral Implants Res. 2011;22(10):1185-1192.
14. Cochran DL, Buser D, ten Bruggenkate CM, et al. The use of reduced healing times on ITI implants with a sandblasted and acid-etched (SLA) surface: early results from clinical trials on ITI SLA implants. Clin Oral Implants Res. 2002;13(2):144-153.
15. Salvi GE, Gallini G, Lang NP. Early loading (2 or 6 weeks) of sandblasted and acid-etched (SLA) ITI implants in the posterior mandible. A 1-year randomized controlled clinical trial. Clin Oral Implants Res. 2004;15(2):142-149.
16. Duddeck D, Maghaireh H, Faber FJ, Neugebauer J. SEM surface analyses of 120 sterile-packed implants. EDI Journal. 2014;10:64-75.
17. Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23(7):844-854.
18. Kasemo B. Biocompatibility of titanium implants: surface science aspects. J Prosthet Dent. 1983;49(6):832-837.
19. González JEG, Mirza-Rosca JC. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J Electroanalytical Chemistry. 1999;471(2):109-115.
20. Delgado-Ruiz R, Romanos G. Potential causes of titanium particle and ion release in implant dentistry: a systematic review. Int J Mol Sci. 2018;19(11):E3585.
21. Weingart D, Steinemann S, Schilli W, et al. Titanium deposition in regional lymph nodes after insertion of titanium screw implants in maxillofacial region. Int J Oral Maxillofac Surg. 1994;23(6 Pt 2):450-452.
22. Wachi T, Shuto T, Shinohara Y, et al. Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology. 2015;327:1-9.
23. Rodrigues DC, Valderrama P, Wilson TG, et al. Titanium corrosion mechanisms in the oral environment: a retrieval study. Materials (Basel). 2013;6(11):5258-5274.
24. Heyman O, Koren N, Mizraji G, et al. Impaired differentiation of Langerhans cells in the murine oral epithelium adjacent to titanium dental implants. Front Immunol. 2018;9:1712.
25. Arizon M, Nudel I, Segev H, et al. Langerhans cells down-regulate inflammation-driven alveolar bone loss. Proc Natl Acad Sci U S A. 2012;109(18):7043-7048.
26. Maiorano E, Favia G. Langerhans cells in periimplantar gingival tissues: an immunohistochemical study of 14 cases. Boll Soc Ital Biol Sper. 1994;70(10-11):257-263.
27. Al-Thobity AM, Kutkut A, Almas K. Microthreaded implants and crestal bone loss: a systematic review. J Oral Implantol. 2017;43(2):157-166.
28. Amid R, Raoofi S, Kadkhodazadeh M, et al. Effect of microthread design of dental implants on stress and strain patterns: a three-dimensional finite element analysis. Biomed Tech (Berl). 2013;58(5):457-467.
29. Ribes N, Angeles FM, Monreal A, et al. Dental implants with different neck designs and surfaces. A 3 year retrospective study. Clin Oral Implants Res. 2017;28(suppl 14):25.
30. Rismanchian M, Birang R, Shahmoradi M, et al. Developing a new dental implant design and comparing its biomechanical features with four designs. Dent Res J (Isfahan). 2010;7(2):70-75.
31. Donley TG, Gillette WB. Titanium endosseous implant-soft tissue interface: a literature review. J Periodontol. 1991;62(2):153-160.
32. Kawahara H, Kawahara D, Mimura Y, et al. Morphologic studies on the biologic seal of titanium dental implants. Report II. In vivo study on the defending mechanism of epithelial adhesions/attachment against invasive factors. Int J Oral Maxillofac Implants. 1998;13(4):465-473.
33. Ericsson I, Persson LG, Berglundh T, et al. Different types of inflammatory reactions in peri-implant soft tissues. J Clin Periodontol. 1995;22(3):255-261.
34. Yeung SC. Biological basis for soft tissue management in implant dentistry. Aust Dent J. 2008;53(suppl 1):S39-S42.
35. Mishra SK, Chowdhary R, Kumari S. Microleakage at the different implant abutment interface: a systematic review. J Clin Diagn Res. 2017;11(6):ZE10-ZE15.
36. Souza AB, Alshihri A, Kämmerer PW, et al. Histological and micro-CT analysis of peri-implant soft and hard tissue healing on implants with different healing abutments configurations. Clin Oral Implants Res. 2018;29(10):1007-1015.
37. Jansen VK, Conrads G, Richter EJ. Microbial leakage and marginal fit of the implant-abutment interface. Int J Oral Maxillofac Implants. 1997;12(4):527-540.
38. Paltop Screw Loosening Test EXT0477 (ISO:18130, ISO:14801, Paltop Platform Sealing Test).